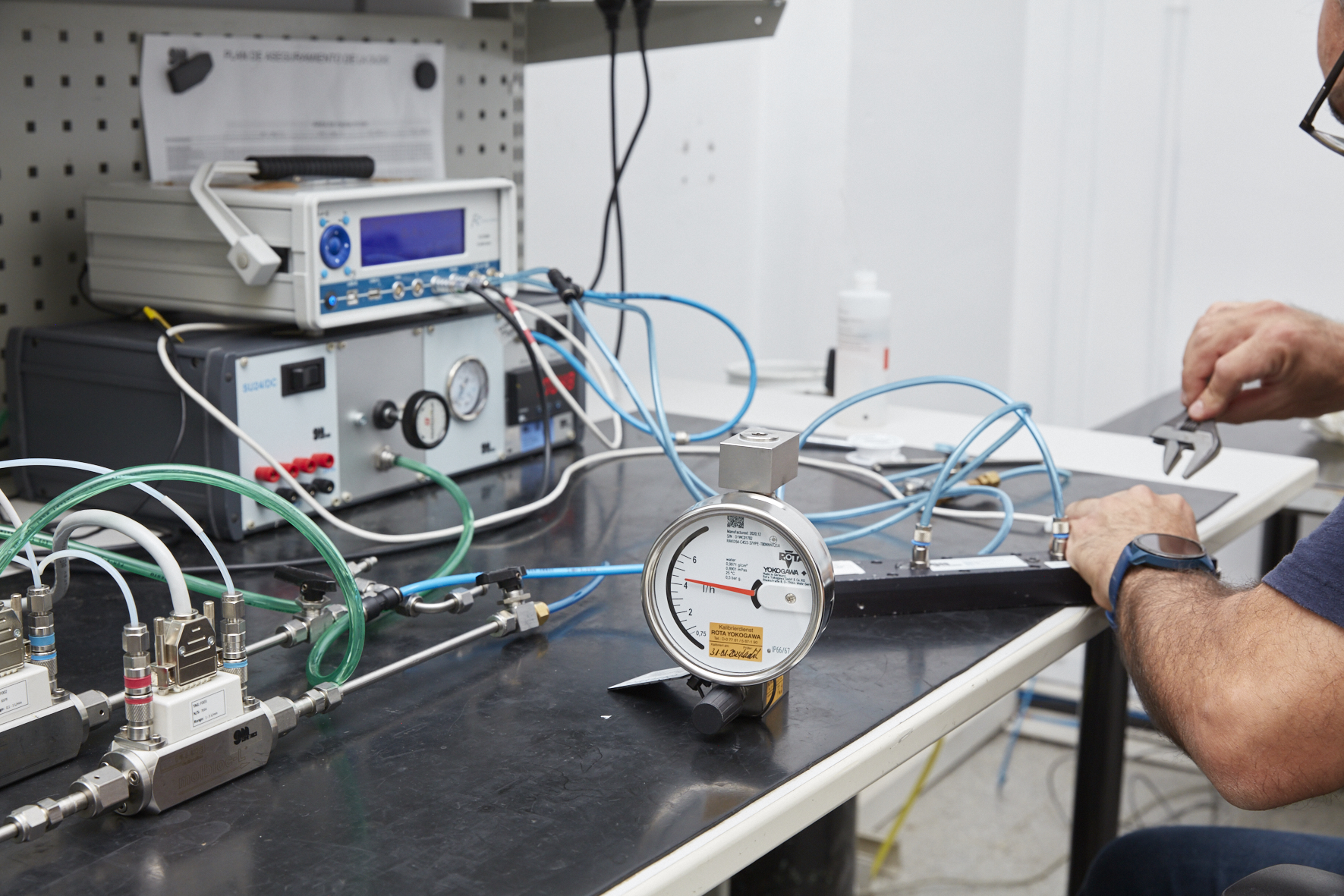
We are often asked about the difference between a factory calibration and a calibration according to UNE-EN ISO/IEC 17025. Selecting one or the other to perform the calibration of measuring instruments will always depend on the type of application and current regulations to be complied with.
Companies in the pharmaceutical, food, automotive or laboratory sectors are obliged to work with equipment calibrated to internationally recognized standards. Regulations such as ISO 9001, GMP or FDA not only recommend it, they demand it.
The most frequent problem is that the meaning and applications for which each of them is used is unknown. We will explain clearly what is the difference between a factory calibration and a calibration according to UNE-EN ISO/IEC 17025, so that you know which one you need, when and why.
What is a factory calibration?
First of all, when we talk about factory calibration we refer to the calibration process performed by the manufacturer during the production of an instrument. The objective is to verify that the new instrument complies with the defined technical specifications and tolerances.
This calibration is documented under a factory calibration certificate, which shows the deviation of the instrument from the standard used.
In the food industry, a balance with a simple factory calibration may be sufficient for basic internal use, such as weighing ingredients to formulate prototypes for new recipes.
But if the same balance is used to determine the net quantity of a packaged product to be sold to the final consumer, the factory certificate would not be valid in the face of an official inspection or audit.
What is a calibration according to UNE-EN ISO/IEC 17025?
On the other hand, calibration according to UNE-EN ISO/IEC 17025 is the calibration performed in an accredited laboratory such as the one we have in our facilities in Barcelona, and which complies with said standard.
This calibration guarantees the traceability of the instrument being calibrated to national standards and to the international system of units (SI). This means that every deviation from the standard is documented for each calibration point, hysteresis and repeatability are also calculated and represented in the expanded uncertainty calculation.
The following process results in the expanded measurement uncertainty.
In many types of applications and industries calibration according to ISO 17025 is mandatory.
In a pharmaceutical cleanroom, differential pressure transmitters measure the pressure difference between rooms of different cleanliness classifications. Maintaining a stable, controlled pressure prevents particle ingress and ensures that air flows follow the validated design.
For this reason, this equipment must be calibrated according to ISO 17025.
Factory calibration vs. traceable calibration according to ISO 17025: What is the difference?
The main difference is that factory calibration is performed under the manufacturer’s internal procedures (without this calibration being internationally recognized), while calibration according to ISO 17025 is a calibration that guarantees the traceability of the instrument to be calibrated according to internal reference standards.
In other words:
Instrument or process calibrations are divided into two main categories: accredited and non-accredited calibrations.

In calibration, metrological traceability is a very important aspect to be taken into account, in order to face audits, comply with regulations such as FDA, ISO 9001, ISO 17025…
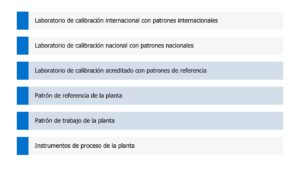
This traceability can be shown as follows. The following image shows the location of the different traceability levels. From the lowest level “In-plant process instruments”, its traceability depends on the higher levels.
The higher we move up the list, the lower the uncertainty or, in other words, the higher the accuracy.
To affirm that a measurement is traceable, a series of events must occur:
- Periodic calibrations:
Calibrations within the traceability chain must be performed at regular intervals. It is not sufficient to calibrate a standard once and continue to use it indefinitely. Each calibration has a period of validity, and when it expires, the associated traceability also expires, since the standards also suffer drifts over time and between calibrations. - Documentation of each step:
Each step in the chain must have a calibration certificate and be supported by written procedures under a quality system. A calibration without a certificate or performed outside a documented system cannot be considered accredited or reliable. - Measurement uncertainty at each step:
It is essential that the measurement uncertainty is documented for each calibration. Without this information, it is not possible to guarantee traceability, since it could happen that a highly accurate instrument is calibrated with a less accurate one, compromising the validity of the measurement.

When do I need an ISO 17025 traceable calibration and when do I need a factory calibration?
It will depend mainly on the regulatory requirements and the type of application required by the instrument or process.
You will only need a factory calibration when using an instrument for internal applications or in processes involving an instrument that do not require regulatory compliance.
On the other hand, you will need an ISO 17025 calibration when you have to comply with international standards, traceability, audits, certifications or when the instrument is involved in processes where measurements must be documented.
In case you still have doubts about which type of calibration best suits your needs, our team will be happy to advise and guide you to choose what best suits your needs.
This content has been reviewed by Toni Esteveresponsible for laboratory and on-site calibration services at Applus+ Gometrics to ensure the accuracy of the information and references.